Focal One® - Robotic Focal HIFU
Landmark HIFI Study Publication Demonstrates Positive Outcomes with Focal One® Robotic HIFU Versus Surgery in the Management of Prostate Cancer
No Surgery, No Cutting, No Radiation
The Focal One® HIFU Robotic System uses high performance, high intensity focused ultrasound (HIFU) technology to precisely target and ablate prostate tissue. By sparing healthy surrounding tissue, it minimizes side effects and helps patients maintain their quality of life.
No Surgery, No Cutting, No Radiation
The Focal One® HIFU Robotic System uses high performance, high intensity focused ultrasound (HIFU) technology to precisely target and ablate prostate tissue. Sparing healthy surrounding tissue, it minimizes side effects and helps patients maintain their quality of life.
Focal One® | Robotic Focal HIFU Treatment
Focal One® | Robotic Focal HIFU Treatment
Non-Invasive Prostate Tissue Ablation – The Focal One® HIFU Robotic System uses high performance, high intensity focused ultrasound (HIFU) technology that allows for precise targeting and destruction of part of the prostate, sparing healthy surrounding tissue and minimizing side effects. By focusing high-intensity ultrasound waves on the affected area, causing localized heating that destroys the cells in the gland without damaging the healthy surrounding tissue. Focused ultrasound works in the same way as rays of sunlight that pass through a magnifying glass and are concentrated at a single point, causing a significant temperature rise around the focal point.
The Key Pillars of the Focal One Platform

Robotic Positioning System
The Focal One robotic positioning system provides submillimetric precision with five degrees of freedom, ensuring accurate targeting while incorporating automatic safety features.
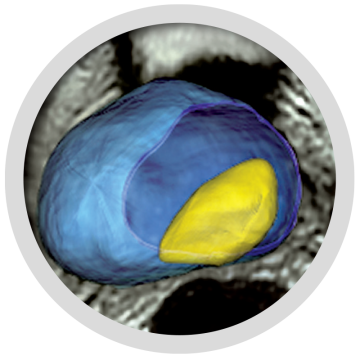
Open-Platform Fusion Software
Fusion HIFUsion® software allows the urologist to import, visualize and perform elastic fusion of MRI or 3D biopsy maps at the time of treatment. This enhances treatment precision by contouring and targeting lesions while avoiding critical structures.

Integrated Workstation
The Focal One cart-based system is designed for flexibility and compatibility with standard hospital OR beds, providing seamless integration into any clinical workflow.

Dynamic Focusing Probe
Dynamic Focusing Probe combines high resolution real-time ultrasound imaging with precise HIFU energy delivery resulting in expanded reach and fastest treatment.
Focal One Advantages

Why Physicians Benefit from a HIFU Program
Why Physicians are Adding Focal One to Their Prostate Cancer Management Programs and How Focal HIFU Therapy Fills an Important Gap in Prostate Cancer Management Programs. Visit the Physician section to review 154 peer-reviewed publications regarding Focal HIFU Therapy.

Why Physicians Benefit From A HIFU Program
Why Physicians are Adding Focal One to Their Prostate Cancer Management Programs and How Focal HIFU Therapy Fills an Important Gap in Prostate Cancer Management Programs. Visit the Physician section to review 154 peer-reviewed publications regarding Focal HIFU Therapy.

How Patients Benefit from HIFU
Discover the Patient Benefits of HIFU and how you can change your prostate health without changing your life. Focal One HIFU Prostate Tissue Ablation allow patients to maintain their quality of life with minimal side-effects. To find a HIFU Center near you, visit the Patient section.

How Patients Benefit From HIFU
Discover the Patient Benefits of HIFU and how you can change your prostate health without changing your life. Focal One HIFU Prostate Tissue Ablation allow patients to maintain their quality of life with minimal side-effects. To find a HIFU Center near you, visit the Patient section.